The Current State of Animal Testing in Beauty - Blog Version
There is so much misinformation about animal testing in the beauty sector, and a lot of opportunistic, probably predatory, marketing to take advantage. In this post, I’ll try to give a thorough overview of the many nuances of the topic. Why we started testing on animals, the scientific investment for alternative study methods, historical animal testing bans, regulatory conflicts, common misconceptions and more. This blog is based off of the podcast I recorded with Barae Jomaa PhD on The Current State of Animal Testing. Big thank you to Barae for contributing your knowledge! For lots more detail, highly recommend tuning in!
Shout out to Annika Lagut PhD from Coherent Beauty for being my trusted proof-reader, and also to Barae for giving this written post a fact check as well!
But first, a rappid fire on Animal Testing in Beauty Misconceptions
If it’s sold in China=animal testing. Nope! As far back as 2014, this hasn’t been true. This is especially untrue now with the 2021 animal testing ammendments. Unpopular opinion, this misconception has been used to openly be racist towards China. Also their delay for banning animal testing wasn’t nefarious - it was to protect their public.
Companies who don’t label themselves “cruelty free” or who don’t have a leaping bunny certification are testing on animals. Nope! Nobody is actively testing their products on animals these days. The regional animal testing bans have had global impact - it makes zero economic (because animal tests are expensive) or business (because consumers don’t want to see it) sense to test cosmetics on animals. IMO, this is a misleading claim because it convinces the public animal testing is still widespread. It is not. Obviously this is my opinion, and there are many people who I have a lot of respect for who disagree with me. Also - what do we mean by cruelty free and who are we considering? Surely workers should be considered too?
All the big beauty brands are testing on animals. Nope! See last section. ALSO, the companies investing the most into the advancement of animal-testing alternatives, and the companies having the most impact in terms of advocating for no animal testing? It’s the big guys.
Why did we start testing on animals? Pt 1. The dark history of Cosmetics Safety
From the 18th century’s use of lead carbonate in makeup to the use of radium in the 1920s and 30s, there have been many deaths in the name of beauty. Many of these practices stemmed from ignorance - we had no idea the safety risks when these products were placed on the market. Historically animals have been used as an indicator of safety. E.g. canary in the coal mine, or royalty using pets to test food for poison.
Why did we start testing on animals? Pt 2. Why not test on humans?
We did. E.g. Nazi germany. Nazi’s cared a lot about animal welfare, so they used people they thought were less important.
This resulted in the Nuremberg Code: a set of ethical research principles for human experimentation.
1st principle - you need consent.
2nd principle - the experiment must be useful to society and not attainable in any other way.
3rd principle - human experiments should be based on animal experiment results.
You don’t want to test on humans (e.g. clinicals) until you have safety confidence. At the time, alternative animal experiments and in-vitro testing didn’t exist. This is the basis for animal safety testing in cosmetics and beyond.
Sadly, this did not end human experimentation. E.g. Kligman’s Acres of Skin from 1951-1974, where prison inmates in Philadelphia underwent non-therapeutic medical experiments.
The logics of animal testing
Other animals are similar to us. Biological complexity that has yet to be replicated in alternative test methods
Shorter life cycles. They can be studied throughout their life span/across generations.
Animal research continues to be required in drug development to demonstrate safety and efficacy before starting human research. This is less relevant to cosmetics, where there are bans. Nonetheless, the cosmetics industry is paving the way to a future with no animal testing with our disproportionate investment into alternative test methods.
When one animal isn’t reliable, two types may be used (e.g. rats and rabbits). Roughly 95% of animals used are rodents that are bred for laboratory use.
Ethics board approval requirements in order to start animal testing.
The OECD sets out testing guidelines, which are predominantly non-animal methods, with animals only used when the non-animal methods aren’t sufficient. They currently have a call to action for the urgent resources needed to support new safety testing method validation in order to reduce the use of animals and better protect human and environmental health. Drug testing methods are set out by the International Conference on Harmonization (ICH). Here, animals predict phase 1 human trial safety with roughly 90% average accuracy.
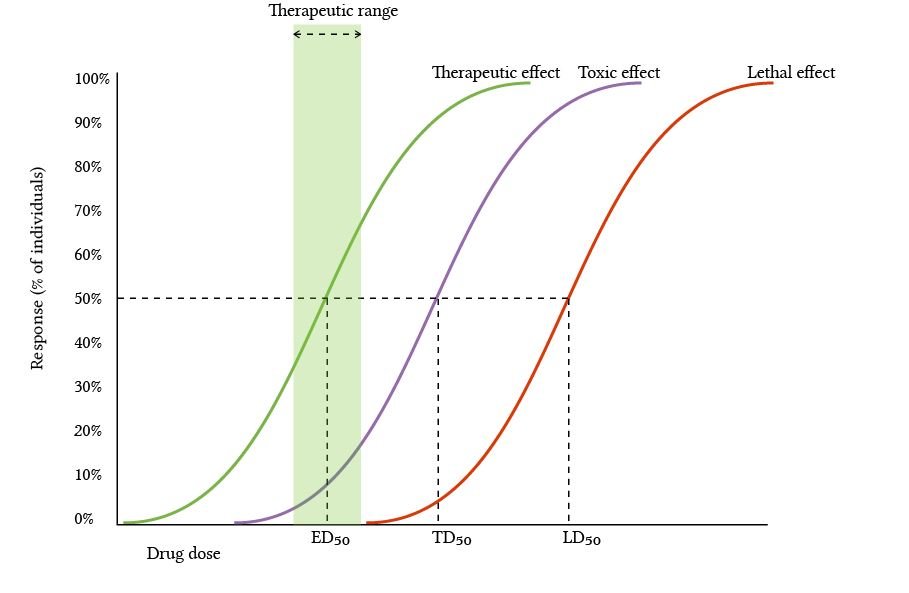
E.g. the LD50 Test: LD stands for “lethal dose”, this is the amount of a substance needed to kill 50% of an animal test group. This test is one way of estimating the acute (short term) toxicity of a substance. After years of controversy, conventional LD50 tests were suspended in 2002, followed by the implementation of OECD TGs, where the number of test animals was reduced to 2-15 animals (from 30 for 3 doses previously).
History of alternative (non-animal) safety testing methods
Kicked off with 1959 publishing of the principles of humane experimental techniques by Russel and Burch; introduced concept of three r’s, reduction, refinement and replacement of animal testing. Laid the foundation.
1961: humane research fund established in the UK to support scientific development of alternatives
1980s: development of the 3D reconstructed skin calls or skin tissue constructs, which allowed for development of many tests that used it for toxicity testing.
1981: first alternative to the Draize test based on ex vivo eyes from slaughterhouses
1990s: development of the epiocular test that’s commonly used as an invitro test based on cell lines - e.g. reconstructed human corneal like epithelial cells in tissue constructs.
Many other tests came after but these were major milestones for the development of alternatives.
Current gaps in alternative testing include ones that involve long term exposure, reproductive and developmental toxicity, systemic exposure and endocrine impacts. In-vitro tests are good at looking at acute toxicity, not great at these longer term, more complex endpoints. There are some tests in the works to address these gaps, but they still need validation. Validation is crucial to understand the accuracy, reliability and reproducibility of a test, if the results reflect what happens in humans, and if it’s viable for wider panel of substances. Developing tests is rather easy in the grande scheme of things, validating is not. Much more research is needed.
Misconception: there are alternatives to every safety test. There are not.
History of Animal Testing Bans
1998 UK ban for cosmetic products and ingredients.
2004 EU ban for finished products
2007 Israel had bans for finished products
2013 full ban on EU ingredients
2014 India starting animal testing ban, China also started implementing new rules to remove mandatory testing of “ordinary” cosmetics manufactured in China.
2015 New Zealand, Turkey, South Korea, Taiwan, Switzerland
2017 Guatemala 2020 California, Colombia
2021 Mexico, Virginia, Maryland, Maine, Hawaii, New Jersey
2021 China expanded removal of mandatory testing for general cosmetics inc those not manufactured in China.
2022 New York, Louisiana
2023 Oregon, Canada, Brazil
Due to historical bans, very little animal testing has happened in cosmetics since the EU full ban on ingredients in 2013. Companies are not producing locally generally, they are producing potentially for the world. Therefore, they have to meet global regulations. Note, animal testing is also expensive and consumers don’t want to see it. No cosmetic company wants to have their products tested on animals. It makes zero economic or business sense anymore.
The alternative testing methods that have been developed in the cosmetics industry will be useful for many other industries, including pharma. The cosmetics industry has invested disproportionately more into non-animal safety testing methods compared to any other sector.
Why all these bans?
Prompted by 1980s activism, famously by Henry Spirra who published an ad about Revlon’s use of the Draize test which forced them to start innovating alternative testing methods. This ad bolstered an activism movement that brought wider public attention to animal testing practices. There was also already interest in the scientific community, i.e. the 1961 Humane Research Fund, to develop non-animal safety testing methods.
The current EC and REACH Conflict
In Europe, REACH set out 3 compliance deadlines for chemicals based on tonnage, the last of which was in 2018. To be registered, chemicals had to undergo a suite of testing to ensure safety, but some of these tests were animal tests that had no acceptable alternatives (reproductive and developmental toxicity).
In parallel, EC cosmetic regulations have an ingredient ban for ingredients tested on animals.
In 2014, ECHA clarified that this would not be required if a chemical is exclusively used in cosmetics. But when it comes to worker safety, you cannot waive the tests. This made it difficult to manage unless the ingredient was produced abroad and imported, giving an advantage to non-EU companies and disadvantage to local companies.
There has been a very messy back and forth regarding this conflict for the last decade, with a lot of petitioning from the cosmetics industry. The cosmetics industry does not want to test on animals, and has invested billions into alternatives. (unfortunately, there is still a gap, see history of alternative methods slide, which is the basis for REACH’s testing requirements.)
Disclaimer, this largely impacts ingredient suppliers, rather than finished product produced (i.e. the brands). Brands can just choose not to use the ingredients impacted, which are only a few - note, much of the ingredients used in beauty have historical, pre-2013 animal safety testing data to support safety.
Disclaimer, this may make it extremely challenging to launch new e.g. preservatives or UV filters in Europe (if it wasn’t already)
Disclaimer, so far ECHA has ordered the testing of certain UV filters, which has resulted in a lawsuit in the company’s defence (Unilever in defence of Symrise against ECHA). The lawsuit was dismissed last year - currently a developing story. At this stage, to the best of my knowledge, no companies have performed animal testing to appease these conflicting regulatory demands.