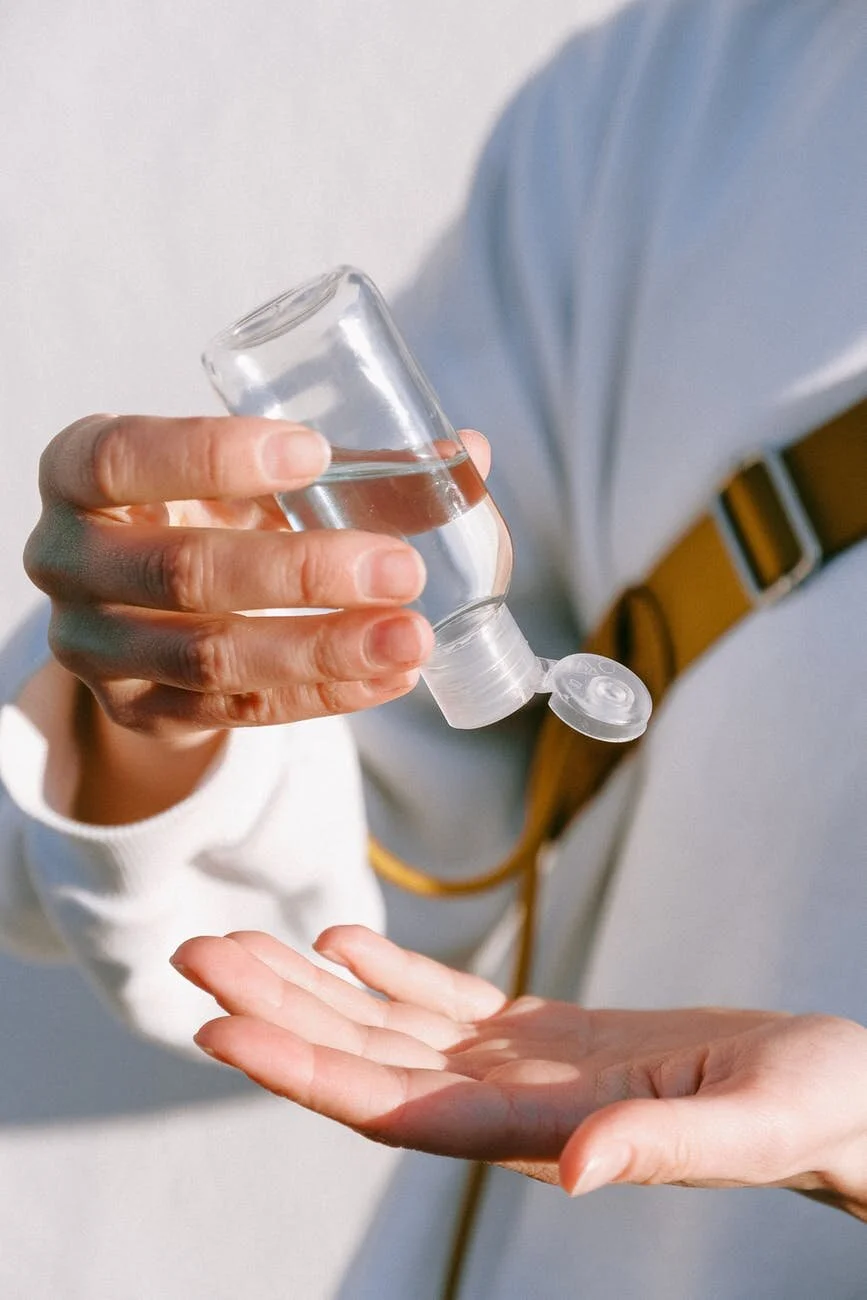
Perspectives on DIY Hand Sanitizers from 5 concerned cosmetic scientists
Amid our current global health crises, there’s no shortage of dangerous misinformation and unproven COVID-19 ‘remedies’ floating around: homeopathy, vitamin C, chiropractic, supplements, essential oils, colloidal silver, and the list goes on. Please consider reporting that shit as it presents a significant public health risk. With this, DIY hand sanitizer recipes have been circulating like wild fire. Unfortunately, most of them will not work. Instead, they may do more harm than good by giving people a false sense of confidence. Unbeknownst to a lot of people, chemists are behind your store-bought products, and formulating is a science. Unless you have a background, you probably aren’t going to get it right (like any science). Most of these recipes come from, unfortunately, people who have no idea what they’re talking about. This blog post featured the perspectives of 5 concerned cosmetic scientists: Tammy Lisi, Dr Michelle Wong, Jane Barber, Dr Anke Ginzburg and myself (Jen).
Overwhelming take home: please just purchase the product from reputable companies, and if you cant, just be more vigilant with your hand washing! PS, If you would just like the quick and dirty for this post, there’s a 2 minute video summary at the end :).
“COVID-19 is caused by SARS-CoV-2 (novel coronavirus 2019). One of the ways this virus spreads is by getting on your hands, which then touch your eyes, nose and mouth, then the virus can take hold and multiply in your respiratory tract. To prevent this from happening, health authorities have been promoting hand washing for at least 20 seconds with soap and water, and hand sanitisers when you can’t wash your hands.” - Dr Michelle Wong, @labmuffinbeautyscience
What is a hand sanitizer and why do they work?
Ultimately they are leave on products intended to be used when soap and water aren’t available to eliminate microbes. Sanitizers can be non-alcohol-based and include benzalkonium chloride (the active ingredient in most alcohol-free sanitizers today - note, as of today, not demonstrated to be effective against SARS-CoV-2) or alcohol based, which is demonstrably effective against SARS-CoV-2. Most alcohol-based hand hand sanitizers have isopropanol (isopropyl alcohol), ethanol, n-propanol, or a combination of 2 of these products.
The effectiveness of alcohol as an antimicrobial is thanks to the fact that it can denature and coagulate proteins - this ultimately leads to microbial cell breakdown. Solutions with 60-95% alcohol are most effective - higher concentrations are less potent because proteins aren’t denatured as easily without water. Water also helps slow the evaporation of the alcohol, which will happen very quickly with 100% alcohol - which means alcohol will have more time to work its magic on your skin. Concentrations with the best results against SARS-CoV-2 for ethanol is 60-70% and for isopropanol (i.e. isopropyl alcohol) around 70% - effects are broad and immediate. Relevant to COVID-19, alcohols used in sanitizers are effective against viruses (just to quickly break down the myth that they aren’t), including SARS-COV-2. *None of these alcohols have shown potential for acquired bacterial resistance.
Regulations surrounding hand sanitizers, by Jane Barber @makingskincare
For a cosmetic product to be sold in the EU, the EU Cosmetics Regulation must be adhered to. The Regulation states, amongst other requirements, that a safety assessment, product information file, required tests and specific labelling must be obtained before the product can be sold. If, however, a product is marketed as a "hand sanitizer" and its primary function is to disinfect, sanitize or kill germs, the product would fall under the definition of a biocide rather than a cosmetic. It would be governed by the Biocidal Products Regulation and must be authorised and active substances and technical documentation verified before being sold. If the product is marketed as treating or preventing a disease or killing or acting on a specific pathogen, for example, COVID-19 it would be classed as a medicine. Laboratory tests, clinical trials and scientific evaluation need to be carried out. Any claim which is made needs to have strong data to back it up. There are international standardized test methods, such as ISO and CEN on killing microorganisms and these must be strictly adhered to.
Note, similarly, in North America these products are regulated as drugs and not as cosmetic products. If a company has complied to regulations (which is very important), you’ll find a DIN number at the back of the package. You may also find an NPN number if you’re in Canada.
NOTE - SEE BELOW FOR A TEMPORARY UPDATE BY THE FDA REGARDING PRODUCTS SOLD IN NORTH AMERICA DURING OUR CURRENT HEALTH CRISIS.
How is a sanitizer supposed to be formulated? By Tammy Lisi @unicornchemist
There are some basics required to make an effective sanitizer.
1 - You want to make sure the alcohol sticks around for long enough and doesn’t just evaporate (contact time) and you want the alcohol at the right concentration.
a) Alcohol requires ‘contact time’ - it takes a little bit of time for the alcohol to denature microbial proteins. b) As mentioned above, 60-70% ethanol and 70% propyl alcohol is the sweet spot to be safe and effective.
2 - Do not burden the sanitizer with extra microbial pressures
Ingredients like Aloe and homemade plant extracts add a microbial burden. If you have amazing proof it doesn't, please forward it to me. Finished products like Aloe Gel that are not properly handled potentially add a microbial burden.
3 - Claims must be substantiated
Unless you’ve tested and demonstrated that your product will ‘kill SARS-COV-2”, do not make the claim. See above for how sanitizers are regulated.
4 - Don’t burn the babies. This is the number one rule of formulations, and I am dead serious about this. Do not, I repeat, do not burn the babies.
a) Essential oils in uncontrolled % risk harmful skin interactions. b) Essential oils in combination with materials that they do not readily mix with such as alcohol and water create a non-homogeneous solution that can again cause harmful skin interactions. c) Repeated use of ingredients like alcohol dry out the skins’ acid barrier which in turn causes irritation that can actually increase the risk of microbial fiesta (this is my favorite description of infection).
Things like polymers are also used to increase contact time and help reduce trans epidermal water loss (TEWL) - these are typically ‘synthetic’ (e.g. carbomers, vinyl acrylates, etc) as ‘natural’ gums (e.g. guar and xanthan) don’t work well for this formulation type. These polymers are not emulsifiers, but rheology modifiers - they help increase thickness, but can also improve contact time for the sanitizer by laying down a microfilm on the skin for example. Since these ingredients typically have to be hydrated then neutralized, and then mixed for a prolonged period of time, most DIYer’s won’t have the materials at home to do this effectively (e.g. a pH meter, overhead mixer, etc). Ingredients like humectants and fragrance (which we would than have to add a solubilizer, so it doesn’t separate over time) are also used in sanitizer products.
Before this magic is released for sale, we run a bunch of testing. 1) Stability to satisfy the FDA’s monograph on hand sanitizer (yes there are rules). 2) Microbial testing and challenge testing against a selection of organisms. 3) Identity and concentration testing on the actives used. 4) Human Repeat Insult Patch Test HRIPT. Verifying that their products do not cause irritation when repeatedly applied to human subjects over time.
Take home - sanitizers are a lot more complex than most consumers realize. Please consider just enjoying your life and let the chemists formulate your products to the best of the science they understand intimately.
A review of some DIY sanitizer recipes floating around and why they won’t work, by Dr Michelle Wong, @labmuffinbeautyscience
Thanks to the COVID-19 pandemic there’s a hand sanitizer shortage… and a ton of DIY hand sanitizer recipes. Will they actually work? (Spoiler: Most of them won’t.)
The WHO has these recommended hand sanitiser recipes - note* only recommended if you can’t purchase a store bought sanitizer (you could also just me more vigilant with your hand washing). If you can, buy the professionally formulated product, it will be safer and better in general. These consist of high alcohol content, glycerin (to soften hands) and hydrogen peroxide (to kill bacterial spores on equipment – this doesn’t act to kill germs on your hands). If you absolutely must make a sanitizer (note - if you can’t find one in store, we recommend using soap and water instead), you need to get over 60% alcohol in the final product. To help you out, I’ve also made an Alcohol Content Calculator for working out the final alcohol concentration for a recipe. Link is here – it should be pretty self-explanatory.
A note from Dr Anke Ginzburg (@dr.ginza) on alcohol %’s:
Reminder - 60% alcohol is the MINIMUM you’ll need for an effective sanitizer (70% for isopropyl alcohol), otherwise it WILL NOT sanitize effectively. CONCENTRATIONS and AMOUNTS are KEY. If you see a sanitizer recipe, ask yourself: what are the exact volumes and what is the alcohol % in the starting contration. Without this information the recipe is useless. Disclaimer: I am not taking into account m/m% and m/v% (more on that below), I am only referring to the v/v% (volume/volume %) and not considering volume contraction (a funky thing alcohol does when mixed with water).
100% alcohol is very rarely available - it ALMOST ALWAYS contains some amount of water because alcohol is hygroscopic (attracts water - even from the air). As a result, it generally is 99% or less. To get to 60% alcohol in a formula, you must calculate it considering the % of alcohol you start with. Rubbing alcohol is roughly 70%, vodka usually 40%, witch hazel 20% (Note, if it’s a distillate, it will have no alcohol*) - note, YOU CAN NOT CREATE A 60% SOLUTION BY STARTING WITH AN ALCOHOL THAT IS LESS THAN 60% ALCOHOL. In a formula, if you’re going to add anything to it past alcohol, the alcohol must be above 60% as the other ingredients will result in the alcohol % sinking (i.e. dillution).
A note from Jane Barber (@makingskincare) on the mass of alcohol:
To throw a wrench into things, when you make alcoholic hand sanitizers using a scale, it’s crucially important you’re aware of the difference between weight and volume if the ethanol concentration of the solution you are using is labelled in volume. Ethanol is commonly sold by volume rather than weight. The specific gravity of ethanol at 20 degrees celsius is 0.789. This means if you bought 70% v/v (un-denatured 100% ethanol) and use it to make a DIY ‘hand sanitizer’ using a scale, the bottle of ethanol solution you are adding to the beaker contains 55.23% w/w ethanol, not 70% w/w.
There’s a reason sanitizer products are regulated as drugs - formulations are more complex than you’d think and it’s super important to make sure it will actually work. Before you go make a hand sanitizer product at home, see if you can buy it instead (from a reputable company). If you absolutely must make a sanitizer (note - hand washing is likely more effective anyway so you could also just be vigilant there), we recommend using the guidelines set out by the WHO.
Friendly reminder friends: Clean your hands often, practice social distancing and please please please stay home if you can (ESPECIALLY if you’re sick but even if you feel fine). Being cautious isn’t just about keeping you safe, it’s about slowing the spread to #flattenthecurve & to protect people who may be more at risk. Note - We know the above can be very effective... For all of the other remedies cropping up online, they will not. If you see information about e.g essential oils, vitamin C, colloidal silver, chiropractic, whatever supplement, etc helping out with COVID-19... please take a moment to report that shit. It’s important now more than ever to spread #factsnotfiction 👊
VIDEO SUMMARY
***edit! regarding benzalkonium chloride from the CDC - "CDC does not have a recommended alternative to hand rub products with greater than 60% ethanol or 70% isopropanol as active ingredients. Benzalkonium chloride, along with both ethanol and isopropanol, is deemed eligible by FDA for use in the formulation of healthcare personnel hand rubs2. However, available evidence indicates benzalkonium chloride has less reliable activity against coronavirus than either of the alcohols3."
Updates/News
Temporary FDA Policy Update in the USA during our current health crisis. (March 21, 2020)
Summary: Because of the current hand sanitizer shortages and the fact that more and more consumers are opting to DIY, which may be ultimately unsafe, the FDA has temporarily updated its policy to allow non-drug approved labs to manufacture the products. The stipulations are as follows: that hand sanitizers must only use United States Pharmacopoeia (USP) grade ingredients in percentages consistent with the World Health Organization (WHO) recommendations (which includes alcohol, glycerol, hydrogen peroxide and sterile distilled water at set %’s). Other ingredients are not to be added as they may impact the quality and potency of the product. Firms must use the most accurate methods of analysis available at the site to verify alcohol content before each batch is released for distribution (e.g. via gas chromatography, alcoholmeter, hydrometer, etc). Products labels must be consistent with the attached labeling in Appendix A, B, C, and D. Firms must register their facility and list these products in the FDA Drug Registration and Listing System. Firms will need to have a way to accept adverse event reports for any products they manufacture, and submit adverse event reports to FDA. For more details, visit the link above. What this may mean for consumers - hold on tight, you’re probably going to see more sanitizer products on the shelves shortly.
References:
Gold NA, Avva U. Alcohol Sanitizer. [Updated 2020 Feb 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513254/
Blog Contributors
Jane Barber
Jane, who owns www.makingskincare.com is a full member of the UK Society of Cosmetic Scientists and has experience formulating for a variety of clients, from major commercial brands such as The Body Shop and Boots to natural and organic start-ups. Jane is also responsible for teaching skincare and haircare formulation for a Cosmetic Science Degree at a UK University. Jane also runs a worldwide formulation consultancy team and heads up formulation discussion groups which total over 40,000 members.
Tammy Lisi
Tammy Lisi has been developing new and innovative formulations for over 25 years. She has extensive experience in new material discovery, physiology, and analytical methodology making her unique in the industry. She has her own consulting firm (@unicornchemist). She is sought after for innovative solutions to everyday problems. She started writing as Unicorn Chemist to help small companies grow.
Michelle Wong
Michelle has a PhD in chemistry and talks about the science behind beauty products at Lab Muffin Beauty Science (labmuffin.com). She also makes YouTube videos (YouTube.com/labmuffinbeautyscience) and is active on Instagram (instagram.com/labmuffinbeautyscience).
Anke Ginzburg
Anke completed her PhD specializing in analytical pharmaceutical chemistry, in collaboration with the Nivea Skin Research institute. She has had many roles throughout her career; including active ingredient extraction/testing, quality assurance, formulation development, and scientific communication management - with the Body Shop in NYC and then Coty in London. She’s an active volunteer with the Society of Cosmetic Science and a visiting lecturer at UAL (Master of cosmetic science at London College of Fashion) and the University of Applied Sciences in OStwestfalen-Lippe in Germany.